The functionality of organic semiconductor devices crucially depends on molecular energies, namely the ionisation energy and the electron affinity. Ionisation energy and electron affinity values of thin films are, however, sensitive to film morphology and composition, making their prediction challenging. In a combined experimental and simulation study on zinc-phthalocyanine and its fluorinated derivatives, we show that changes in ionisation energy as a function of molecular orientation in neat films or mixing ratio in blends are proportional to the molecular quadrupole component along the pi-pi stacking direction. We apply these findings to organic solar cells and demonstrate how the electrostatic interactions can be tuned to optimise the energy of the charge-transfer state at the donor−acceptor interface and the dissociation barrier for free charge carrier generation. The confirmation of the correlation between interfacial energies and quadrupole moments for other materials indicates its relevance for small molecules and polymers.
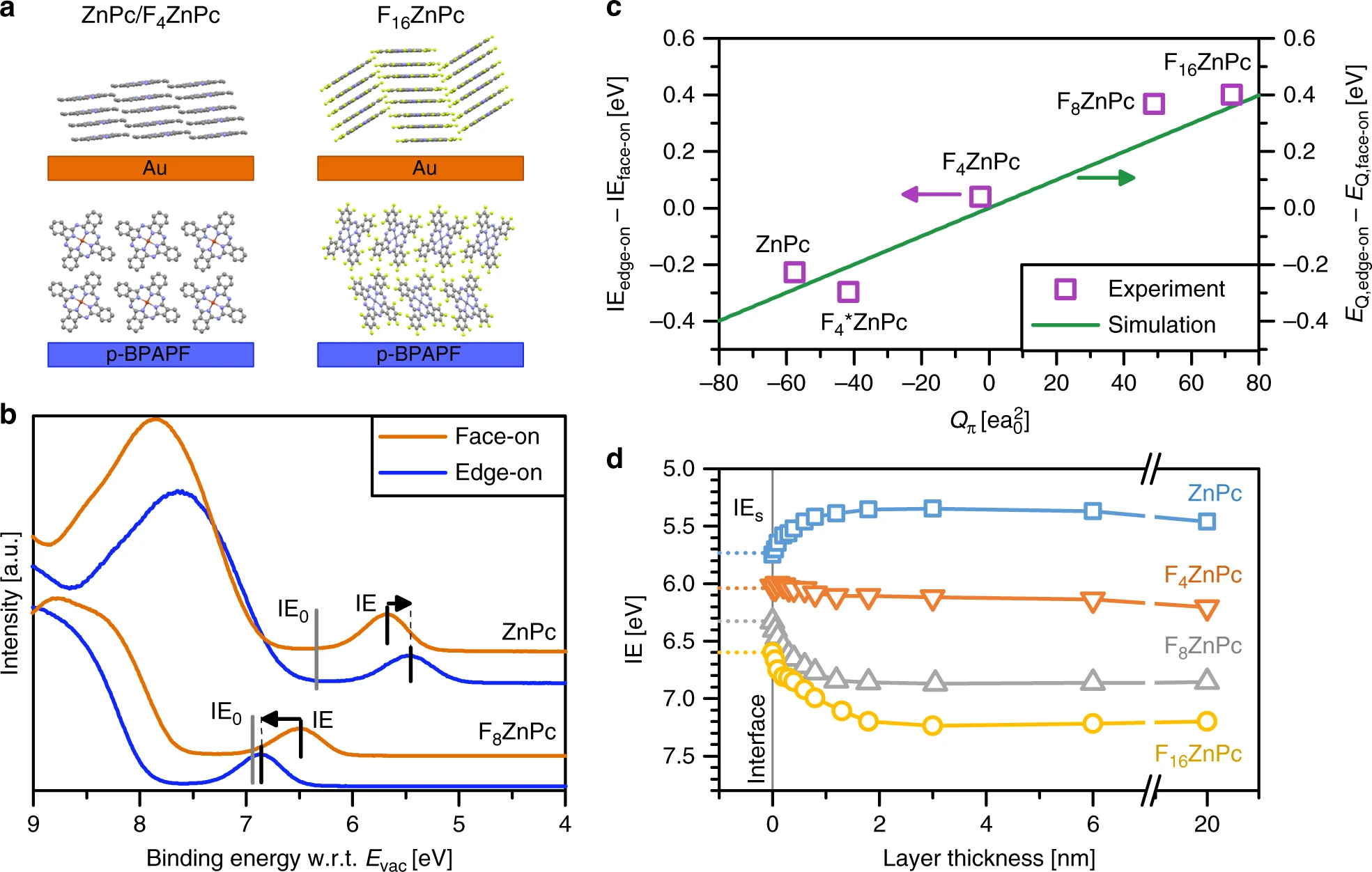
The functionality of organic semiconductor devices crucially depends on molecular energies, namely the ionisation energy and the electron affinity. Ionisation energy and electron affinity values of thin films are, however, sensitive to film morphology and composition, making their prediction challenging. In a combined experimental and simulation study on zinc-phthalocyanine and its fluorinated derivatives, we show that changes in ionisation energy as a function of molecular orientation in neat films or mixing ratio in blends are proportional to the molecular quadrupole component along the pi-pi stacking direction. We apply these findings to organic solar cells and demonstrate how the electrostatic interactions can be tuned to optimise the energy of the charge-transfer state at the donor−acceptor interface and the dissociation barrier for free charge carrier generation. The confirmation of the correlation between interfacial energies and quadrupole moments for other materials indicates its relevance for small molecules and polymers.
