Nature Communications
14, 8313 (2023).
A. Prasoon, X. Yu, M. Hambsch, D. Bodesheim, K. Liu, A. Zacarias, N. N. Nguyen, T. Seki, A. Dianat, A. Croy, G. Cuniberti, P. Fontaine, Y. Nagata, S. C. B. Mannsfeld, R. Dong, M. Bonn, and X. Feng.
Journal DOI:
https://doi.org/10.1038/s41467-023-44129-7
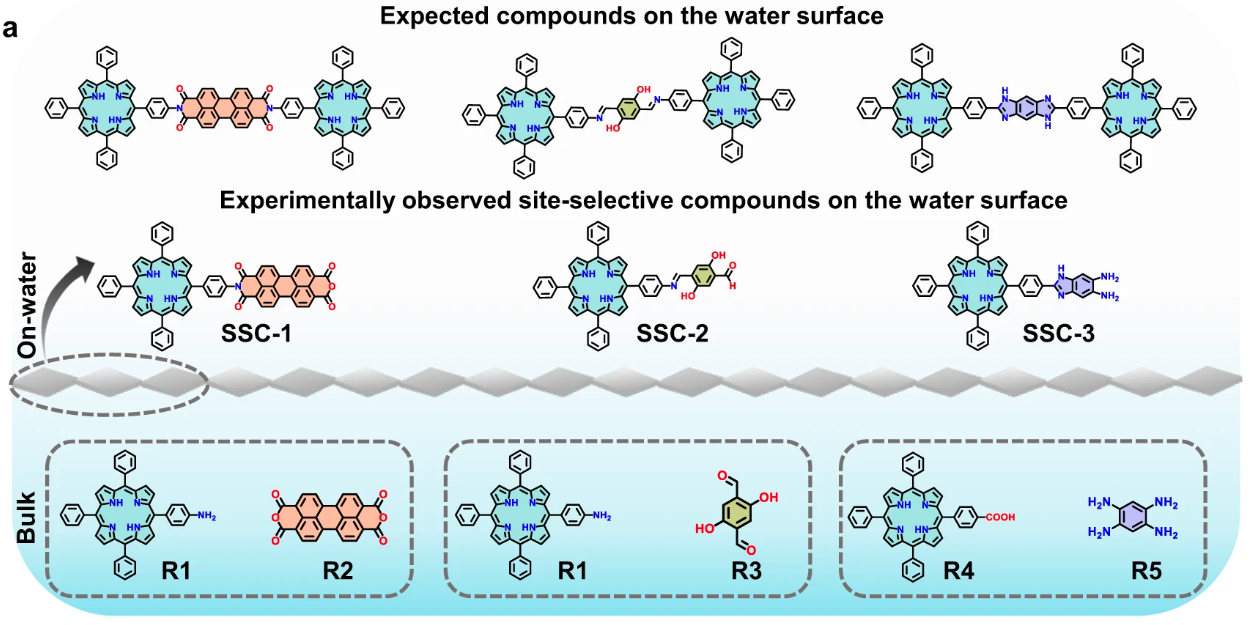
AbstractControlling site-selectivity and reactivity in chemical reactions continues to be a key challenge in modern synthetic chemistry. Here, we demonstrate the discovery of site-selective chemical reactions on the water surface via a sequential assembly approach. A negatively charged surfactant monolayer on the water surface guides the electrostatically driven, epitaxial, and aligned assembly of reagent amino-substituted porphyrin molecules, resulting in a well-defined J-aggregated structure. This constrained geometry of the porphyrin molecules prompts the subsequent directional alignment of the perylenetetracarboxylic dianhydride reagent, enabling the selective formation of a one-sided imide bond between porphyrin and reagent. Surface-specific in-situ spectroscopies reveal the underlying mechanism of the dynamic interface that promotes multilayer growth of the site-selective imide product. The site-selective reaction on the water surface is further demonstrated by three reversible and irreversible chemical reactions, such as imide-, imine-, and 1, 3-diazole (imidazole)- bonds involving porphyrin molecules. This unique sequential assembly approach enables site-selective chemical reactions that can bring on-water surface synthesis to the forefront of modern organic chemistry.