For manufacturing copper interconnects by the damscence technique, electrochemical deposition of copper on patterned sustrates requires several additives to achieve compact filling of trenches and vias, where chloride ions play a crucial role. In the highly acidic electrolyte, adsorption of chloride ions on copper is expected to compete with the adsorption of hydrogen, depending on the copper electrode potential. We propose a general phenomenological model of the coadsorption of two ion species which is supported by DFT calculations and show how the adsorption of one species can be described by the common Langmuir model with rescaled parameters depending on the concentration of the second species. Regarding the Cl- -H+-system, corresponding model parameters are estimated by fitting radio tracer measurements of the chloride adsorption on copper reported in the literature. The data suggest that in a highly acidic solution (pH approximate to 0) the saturation surface density of chloride depends strongly on the electrode potential. With variation of the potential E-SHE from -0.4 to +0.2 V, the saturation density changes by a factor of four. Within our model, such a potential dependence of the saturation density is explained by the presence of adsorbed hydrogen.
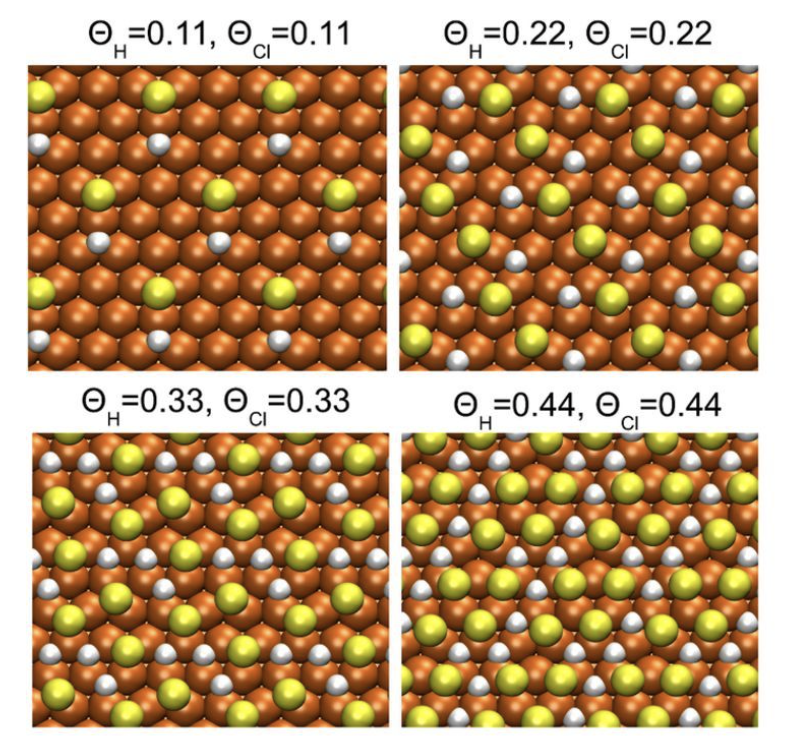
For manufacturing copper interconnects by the damscence technique, electrochemical deposition of copper on patterned sustrates requires several additives to achieve compact filling of trenches and vias, where chloride ions play a crucial role. In the highly acidic electrolyte, adsorption of chloride ions on copper is expected to compete with the adsorption of hydrogen, depending on the copper electrode potential. We propose a general phenomenological model of the coadsorption of two ion species which is supported by DFT calculations and show how the adsorption of one species can be described by the common Langmuir model with rescaled parameters depending on the concentration of the second species. Regarding the Cl- -H+-system, corresponding model parameters are estimated by fitting radio tracer measurements of the chloride adsorption on copper reported in the literature. The data suggest that in a highly acidic solution (pH approximate to 0) the saturation surface density of chloride depends strongly on the electrode potential. With variation of the potential E-SHE from -0.4 to +0.2 V, the saturation density changes by a factor of four. Within our model, such a potential dependence of the saturation density is explained by the presence of adsorbed hydrogen.
