We report on the synthesis and self-assembly of three novel lipophilic guanosine derivatives exposing a ferrocene moiety in the C(5') position of the sugar unit. Their self-association in solution, and at the solid/liquid interface, can be tuned by varying the size and nature of the C(8)-substituent, leading to the generation of either G-ribbons, lamellar G-dimer based arrays or the G4 cation-free architectures.
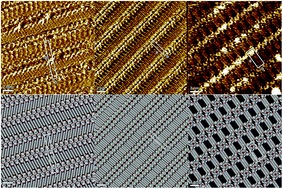
We report on the synthesis and self-assembly of three novel lipophilic guanosine derivatives exposing a ferrocene moiety in the C(5') position of the sugar unit. Their self-association in solution, and at the solid/liquid interface, can be tuned by varying the size and nature of the C(8)-substituent, leading to the generation of either G-ribbons, lamellar G-dimer based arrays or the G4 cation-free architectures.
