In disordered organic semiconductors, the transfer of a rather localized charge carrier from one site to another triggers a deformation of the molecular structure quantified by the intramolecular relaxation energy. A similar structural relaxation occurs upon population of intermolecular charge-transfer (CT) states formed at organic electron donor (D)-acceptor (A) interfaces. Weak CT absorption bands for D A complexes occur at photon energies below the optical gaps of both the donors and the C-60 acceptor as a result of optical transitions from the neutral ground state to the ionic CT state. In this work, we show that temperature-activated intramolecular vibrations of the ground state play a major role in determining the line shape of such CT absorption bands. This allows us to extract values for the relaxation energy related to the geometry change from neutral to ionic CT complexes. Experimental values for the relaxation energies of 20 D:C-60 CT complexes correlate with values calculated within density functional theory. These results provide an experimental method for determining the polaron relaxation energy in solid-state organic D-A blends and show the importance of a reduced relaxation energy, which we introduce to characterize thermally activated CT processes.
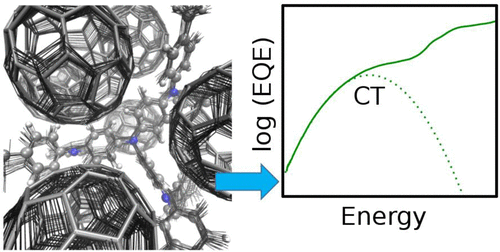
In disordered organic semiconductors, the transfer of a rather localized charge carrier from one site to another triggers a deformation of the molecular structure quantified by the intramolecular relaxation energy. A similar structural relaxation occurs upon population of intermolecular charge-transfer (CT) states formed at organic electron donor (D)-acceptor (A) interfaces. Weak CT absorption bands for D A complexes occur at photon energies below the optical gaps of both the donors and the C-60 acceptor as a result of optical transitions from the neutral ground state to the ionic CT state. In this work, we show that temperature-activated intramolecular vibrations of the ground state play a major role in determining the line shape of such CT absorption bands. This allows us to extract values for the relaxation energy related to the geometry change from neutral to ionic CT complexes. Experimental values for the relaxation energies of 20 D:C-60 CT complexes correlate with values calculated within density functional theory. These results provide an experimental method for determining the polaron relaxation energy in solid-state organic D-A blends and show the importance of a reduced relaxation energy, which we introduce to characterize thermally activated CT processes.
