The realization of a train of molecule-gears working under the tip of a scanning tunneling microscope (STM) requires a stable anchor of each molecule to the metal surface. Such an anchor can be promoted by a radical state of the molecule induced by a dissociation reaction. Our results, rationalized by density functional theory calculations, reveal that such an open radical state at the core of star-shaped pentaphenylcyclopentadiene (PPCP) favors anchoring. Furthermore, to allow the transmission of motion by STM manipulation, the molecule-gears should be equipped with specific groups facilitating the tip–molecule interactions. In our case, a tert-butyl group positioned at one tooth end of the gear benefits both the tip-induced manipulation and the monitoring of rotation. With this optimized molecule, we achieve reproducible and stepwise rotations of the single gears and transmit rotations for up to three interlocked units.
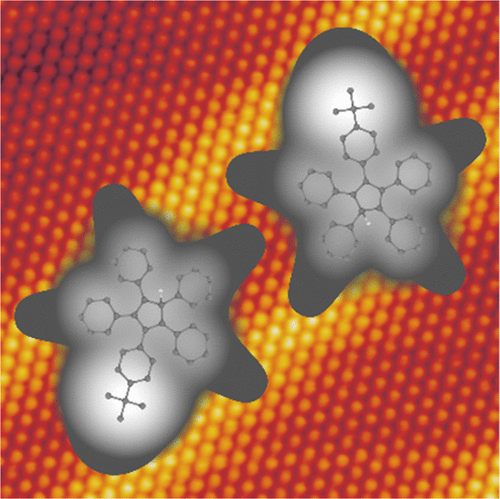
The realization of a train of molecule-gears working under the tip of a scanning tunneling microscope (STM) requires a stable anchor of each molecule to the metal surface. Such an anchor can be promoted by a radical state of the molecule induced by a dissociation reaction. Our results, rationalized by density functional theory calculations, reveal that such an open radical state at the core of star-shaped pentaphenylcyclopentadiene (PPCP) favors anchoring. Furthermore, to allow the transmission of motion by STM manipulation, the molecule-gears should be equipped with specific groups facilitating the tip–molecule interactions. In our case, a tert-butyl group positioned at one tooth end of the gear benefits both the tip-induced manipulation and the monitoring of rotation. With this optimized molecule, we achieve reproducible and stepwise rotations of the single gears and transmit rotations for up to three interlocked units.
