Noble-metal aerogels are emerging functional porous materials that have been applied in diverse fields. Among them, gold (Au) aerogels have displayed grand potentials in a wide range of catalytic processes. However, current fabrication methods fall short in obtaining Au gels with small ligament sizes and controlled surface valence states, which hinder the study of the underlying catalytic mechanisms. Here, a new approach of producing Au aerogels is reported. Via a two-phase ligand exchange, the long-chain ligands (oleylamine) of the as-prepared Au nanoparticles were replaced by short sulfide ions and subsequently self-assembled into three-dimensional gels. As a result, Au aerogels with small ligament sizes (ca. 3-4 nm) and tunable surface valence states are acquired. Taking the application for peroxidase mimics as an example, by correlating the surface valence with the catalytic properties, Au(I) is found to be the active site for H2O2 and substrate-binding site for 3,3′,5,5′-tetramethylbenzidine, paving a new avenue for on-target devising Au-based catalysts.
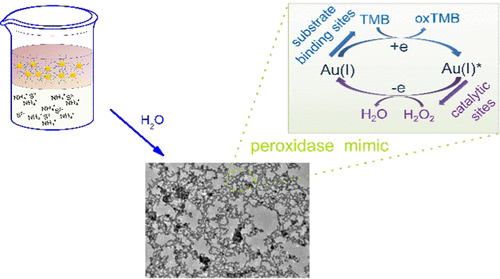
Noble-metal aerogels are emerging functional porous materials that have been applied in diverse fields. Among them, gold (Au) aerogels have displayed grand potentials in a wide range of catalytic processes. However, current fabrication methods fall short in obtaining Au gels with small ligament sizes and controlled surface valence states, which hinder the study of the underlying catalytic mechanisms. Here, a new approach of producing Au aerogels is reported. Via a two-phase ligand exchange, the long-chain ligands (oleylamine) of the as-prepared Au nanoparticles were replaced by short sulfide ions and subsequently self-assembled into three-dimensional gels. As a result, Au aerogels with small ligament sizes (ca. 3-4 nm) and tunable surface valence states are acquired. Taking the application for peroxidase mimics as an example, by correlating the surface valence with the catalytic properties, Au(I) is found to be the active site for H2O2 and substrate-binding site for 3,3′,5,5′-tetramethylbenzidine, paving a new avenue for on-target devising Au-based catalysts.
