Porphyrins, phthalocayanines and their derivatives have found interesting applications in various fields such as molecular electronics, optoelectronics, and sensorics. Common to this class of molecules with a metal-free core is the existence of hydrogen tautomerization reactions. Since the reaction only involves the motion of a pair of hydrogen atoms, it provides a rather simple, yet non-trivial test bed for advanced simulation tools. In this investigation, we exploit state-of-the-art quantum Metadynamics simulations complemented with Nudged Elastic Band (NEB) calculations, to study the effect of structural symmetry on the proton transfer tautomerism of functionalized porphyrins and porphyrazines. Calculated activation barriers using Metadynamics and NEB show a rather good quantitative agreement. We also demonstrate that the set of chosen collective variables in the Metadynamics simulations plays an important role for the appropriate description of the reaction path and dynamics of the system.
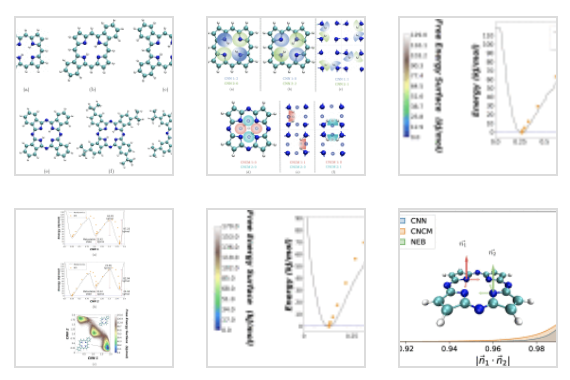
Porphyrins, phthalocayanines and their derivatives have found interesting applications in various fields such as molecular electronics, optoelectronics, and sensorics. Common to this class of molecules with a metal-free core is the existence of hydrogen tautomerization reactions. Since the reaction only involves the motion of a pair of hydrogen atoms, it provides a rather simple, yet non-trivial test bed for advanced simulation tools. In this investigation, we exploit state-of-the-art quantum Metadynamics simulations complemented with Nudged Elastic Band (NEB) calculations, to study the effect of structural symmetry on the proton transfer tautomerism of functionalized porphyrins and porphyrazines. Calculated activation barriers using Metadynamics and NEB show a rather good quantitative agreement. We also demonstrate that the set of chosen collective variables in the Metadynamics simulations plays an important role for the appropriate description of the reaction path and dynamics of the system.
