In order to deploy detonation nanodiamonds (NDs) in nanomedicine and drug delivery applications, fundamental understanding of their surface chemistry and drug loading capacity is highly desirable. Herein, bone morphogenetic protein 2 (BMP-2) derived peptide with the sequence KIPKASSVPTELSAISTLYLGGC (molecular weight = 2336 g/mol) have been successfully grafted onto NDs using carbodiimide crosslinker chemistry. Initially, functional surface groups of wet- and dry-oxidized NDs were compared using infrared and mass spectroscopy. Dry-oxidized NDs exhibited the highest amount of carboxylic acid derivatives with a surface loading of 0.113 mmol/g after air annealing at 415C for 5 hours as determined by mass spectroscopy. Compared to wet-oxidation, the dry-oxidation process showed a 1.4-fold increased amount of carbonyl compounds deduced from infrared and mass spectroscopy. Crosslinking of the carboxylic acid derivatives to the primary amines of BMP-2 derived peptide is feasible in the range of 30-96 percent total surface coverage of NDs. 1:1 and 5:1 ND-peptide ratios were utilized to study the surface loading of NDs using fluorescence spectroscopy. The chemisorption of BMP-2 derived peptide onto NDs reveals superior surface coverage and results in reproducible amounts of tethered bioactive molecules.
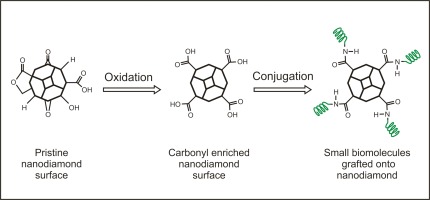
In order to deploy detonation nanodiamonds (NDs) in nanomedicine and drug delivery applications, fundamental understanding of their surface chemistry and drug loading capacity is highly desirable. Herein, bone morphogenetic protein 2 (BMP-2) derived peptide with the sequence KIPKASSVPTELSAISTLYLGGC (molecular weight = 2336 g/mol) have been successfully grafted onto NDs using carbodiimide crosslinker chemistry. Initially, functional surface groups of wet- and dry-oxidized NDs were compared using infrared and mass spectroscopy. Dry-oxidized NDs exhibited the highest amount of carboxylic acid derivatives with a surface loading of 0.113 mmol/g after air annealing at 415C for 5 hours as determined by mass spectroscopy. Compared to wet-oxidation, the dry-oxidation process showed a 1.4-fold increased amount of carbonyl compounds deduced from infrared and mass spectroscopy. Crosslinking of the carboxylic acid derivatives to the primary amines of BMP-2 derived peptide is feasible in the range of 30-96 percent total surface coverage of NDs. 1:1 and 5:1 ND-peptide ratios were utilized to study the surface loading of NDs using fluorescence spectroscopy. The chemisorption of BMP-2 derived peptide onto NDs reveals superior surface coverage and results in reproducible amounts of tethered bioactive molecules.
