Cyano-substituted polyphenylene vinylenes (PPVs) have been the focus of research for several decades owing to their interesting optoelectronic properties and potential applications in organic electronics. With the advent of organic two-dimensional (2D) crystals, the question arose as to how the chemical and optoelectronic advantages of PPVs evolve in 2D compared with their linear counterparts. In this work, we present the efficient synthesis of two novel 2D fully sp 2 -carbon-linked crystalline PPVs and investigate the essentiality of inorganic bases for their catalytic formation. Notably, among all bases screened, cesium carbonate (Cs 2 CO 3 ) plays a crucial role and enables reversibility in the first step with subsequent structure locking by formation of a C=C double bond to maintain crystallinity, which is supported by density functional theory (DFT) calculations. A quantifiable energy diagram of a “quasi-reversible reaction” is proposed, which allows the identification of further suitable C−C bond formation reactions for 2D polymerizations. Moreover, the narrowing of the HOMO-LUMO gap is delineated by expanding the conjugation into two dimensions. To enable environmentally benign processing, the post-modification of 2D PPVs is further performed, which renders stable dispersions in the aqueous phase.
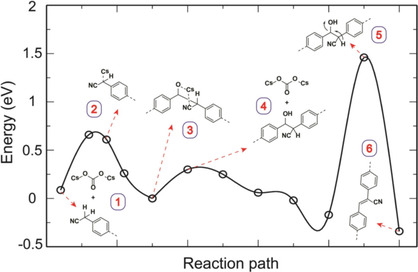
Cyano-substituted polyphenylene vinylenes (PPVs) have been the focus of research for several decades owing to their interesting optoelectronic properties and potential applications in organic electronics. With the advent of organic two-dimensional (2D) crystals, the question arose as to how the chemical and optoelectronic advantages of PPVs evolve in 2D compared with their linear counterparts. In this work, we present the efficient synthesis of two novel 2D fully sp 2 -carbon-linked crystalline PPVs and investigate the essentiality of inorganic bases for their catalytic formation. Notably, among all bases screened, cesium carbonate (Cs 2 CO 3 ) plays a crucial role and enables reversibility in the first step with subsequent structure locking by formation of a C=C double bond to maintain crystallinity, which is supported by density functional theory (DFT) calculations. A quantifiable energy diagram of a “quasi-reversible reaction” is proposed, which allows the identification of further suitable C−C bond formation reactions for 2D polymerizations. Moreover, the narrowing of the HOMO-LUMO gap is delineated by expanding the conjugation into two dimensions. To enable environmentally benign processing, the post-modification of 2D PPVs is further performed, which renders stable dispersions in the aqueous phase.
